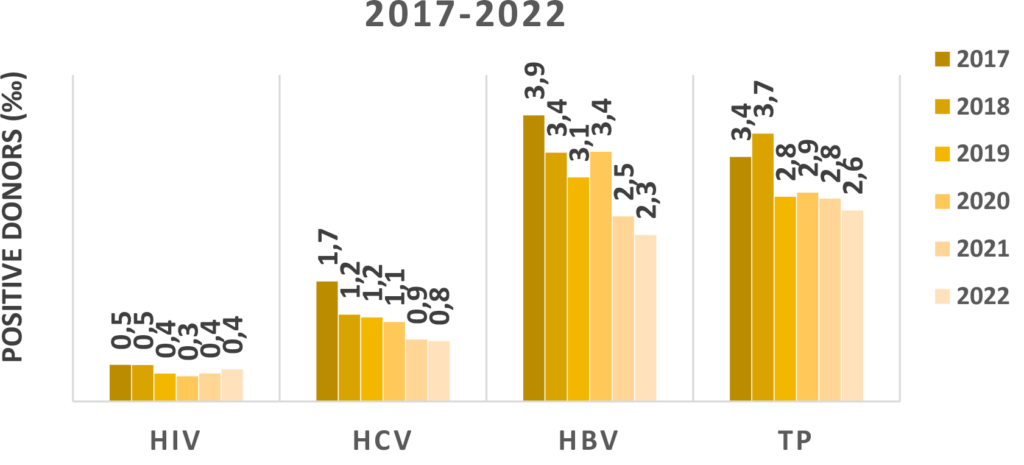
Epidemiological surveillance of transfusion-transmissible diseases is the tool used for the evaluation of the safety of donated blood and blood components. Currently, the biological qualification tests carried out on donors consist in searching for serological markers and the viral genome for Hepatitis B Virus (HBV), Hepatitis C Virus (HCV) and AIDS (Human Immunodeficiency Virus, HIV) infections, and in searching for the serological marker for Syphilis, as established by the Ministerial Decree of 2nd November 2015.
Surveillance of the donor population as regards infectious diseases is an indispensable tool to monitor the national epidemiological situation and to assess the efficiency of the instruments used in the screening of blood and blood components in the Transfusion Services (TS).