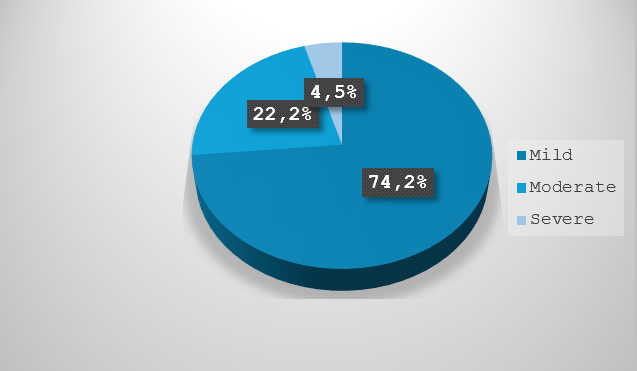
Adverse reactions in donors are unexpected responses that may occur during the blood or blood- component collection process, which are severe enough to be life-threatening, cause death, or are disabling/incapacitating to the donor. The notification of such reactions to the competent authorities became mandatory under Legislative Decree No. 261 of 20th December 2007. 261. The notification forms were developed in accordance with the classification proposed by the International Society of Blood Transfusion/European Haemovigilance Network (ISBT-EHN), both in terms of type and degree of severity and ascribability to the donation procedure The Italian Transfusion Services Information System (SISTRA) not only processes data relating to this section at both regional and national aggregate level, but also classifies the events by type and severity and associates them to different types of both autologous and homologous donation. The monitoring of reactions that may occur in donors is a useful tool for assessing the effectiveness and safety of donor selection protocols and collection procedures for whole blood and apheresis donation.